Our research aims to combine cutting edge techniques with questions about brain hemodynamics regualtion to mechanistically dissect cell types and the signaling pathways responsible for this control. Below are some of the techniques we regularly incorporate into our research to answer these questions.
Want to know more about how to apply each technique to our experiments in awake, behaving mice? See Zhang et al., Neurophotonics, 2022 Online or PDF.
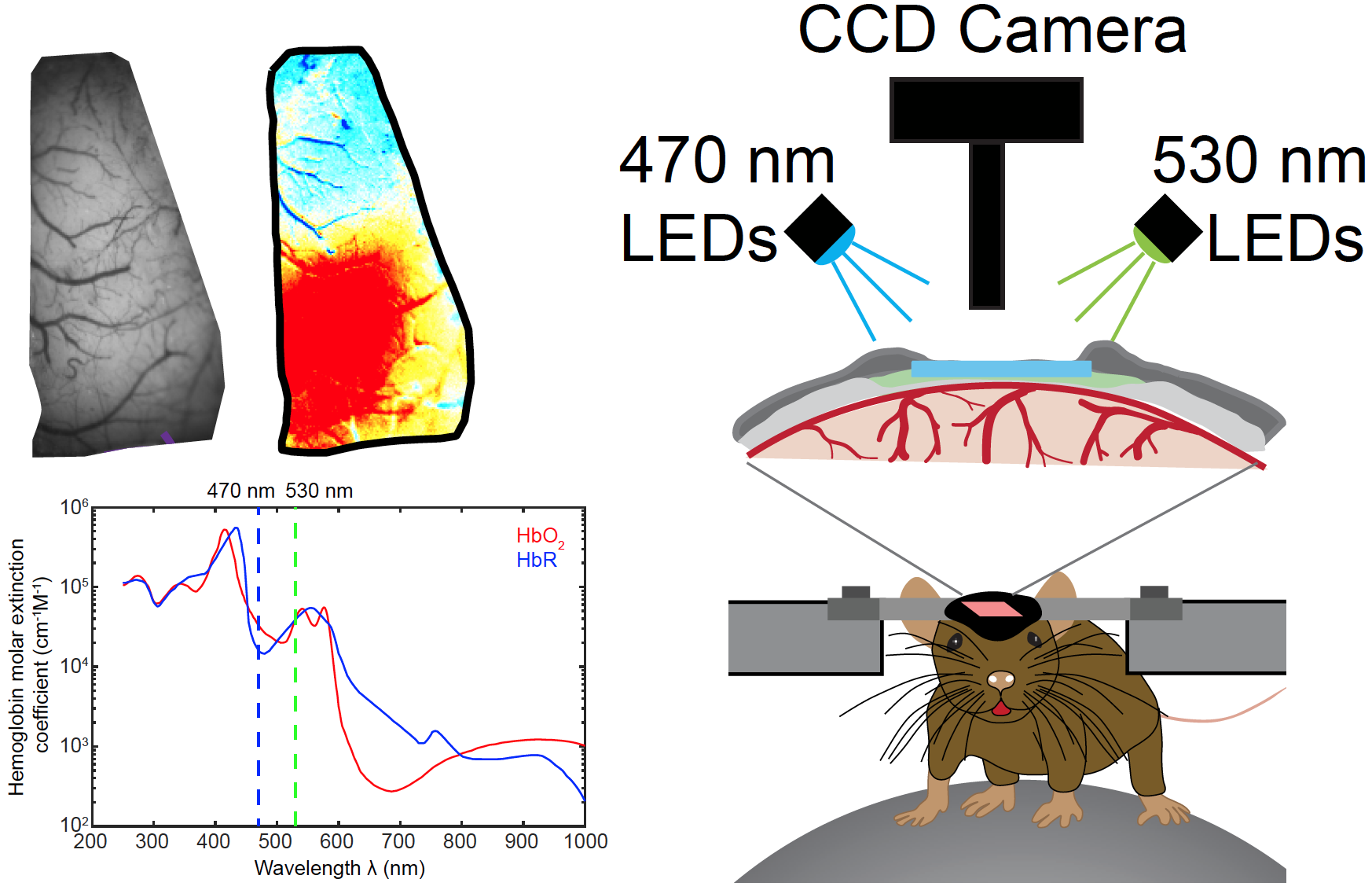
Intrinsic Optical Signal (IOS) Imaging
IOS imaging takes advantage of the spectral properties of hemoglobin, which has different absorption properties when oxygenated or deoxygenated. The basic principle of IOS imaging is that when brain tissue is directly illuminated, the active areas reflect less light than non‑active areas. The more active a brain area the less light reflected, making highly active areas appear darker. There are three main reasons why neuronal tissues change their optical properties when active: active neurons scatter more light (due to swelling and ion movements), natural fluorophores within the cells react (including haemoglobin) and the changes in blood volume/oxidation.

Two-photon Laser Scaning Microscopy (2PLSM)
2PLSM is a fluorescence imaging technique that allows the visualization of living tissue at depths unachievable with conventional one photon flurescence microscopy. It relies upon the principle of two-photon absorption. The key benefit of 2PLSM is its ability to restrict excitation to a tiny focal volume in thick samples, which reduces the out of focus excitation and phototoxicity. An additional benefit of 2PLSM occurs through the use of infrared wavelengths, which reduces the scattering and allows to image deeper in the tissue.

Fiber Photometry Calcium Imaging
Fiber photometery uses the genetically encoded calcium indicator (e.g., GCaMP) to record the neural activity of genetically defined subpopulations of neurons through an optic fiber in freely moving animals. We use this technique to record fluorescence signal from differnt cell types in free behaving mice. This allows us to understand the function of certain cell types in regulating brain hemodynamics and behavior.
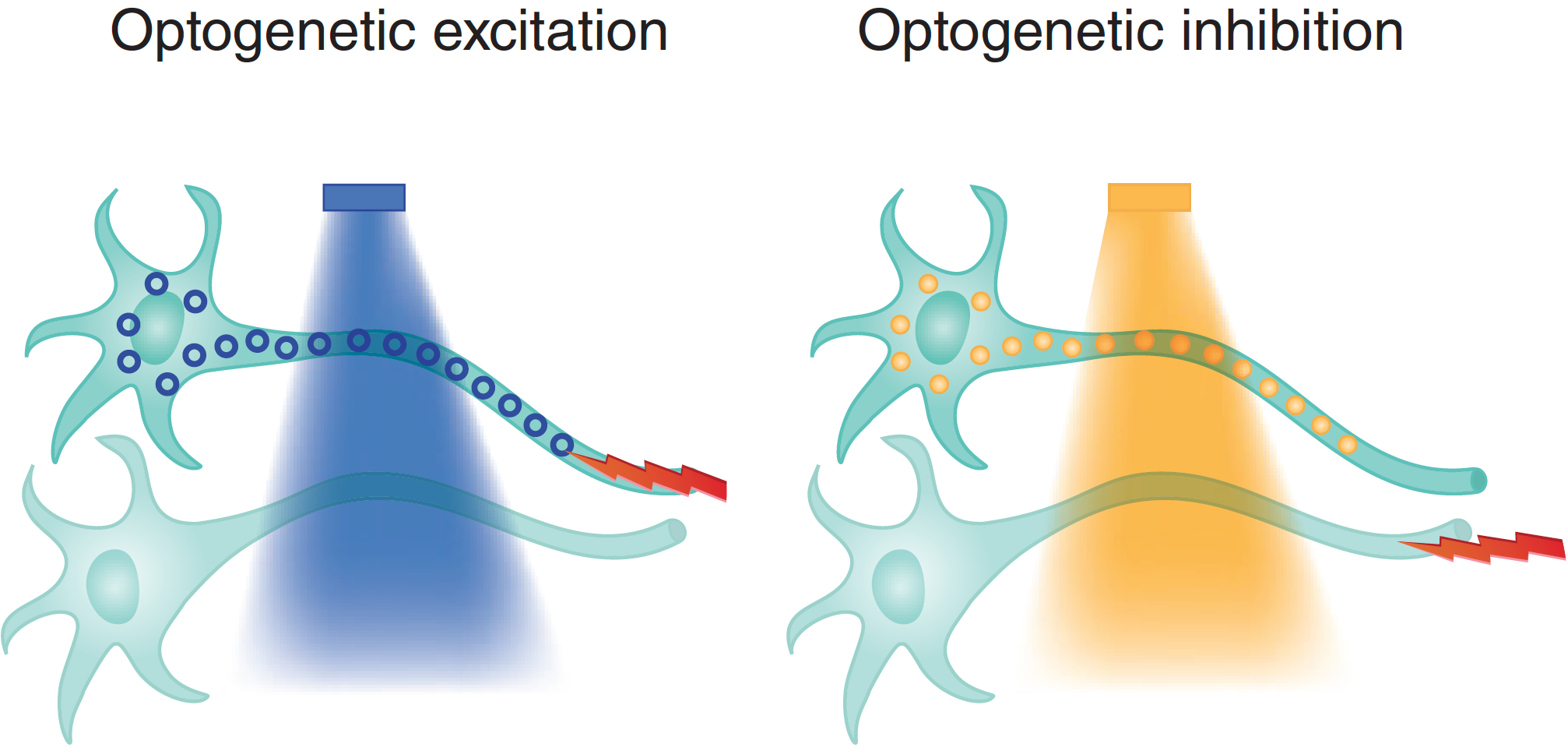
Optogenetics
This technique utilizes light activiated ion channels to induce action potential excitation (or inhibition) in neuronal subpopulations. Using channelrhodopsin, we can temporally and spatially specifically activate defined populations of neurons in vivo. We combine optogenetics with optical imaging to understand the role of defined cell types in brain hemodynamics regulation.
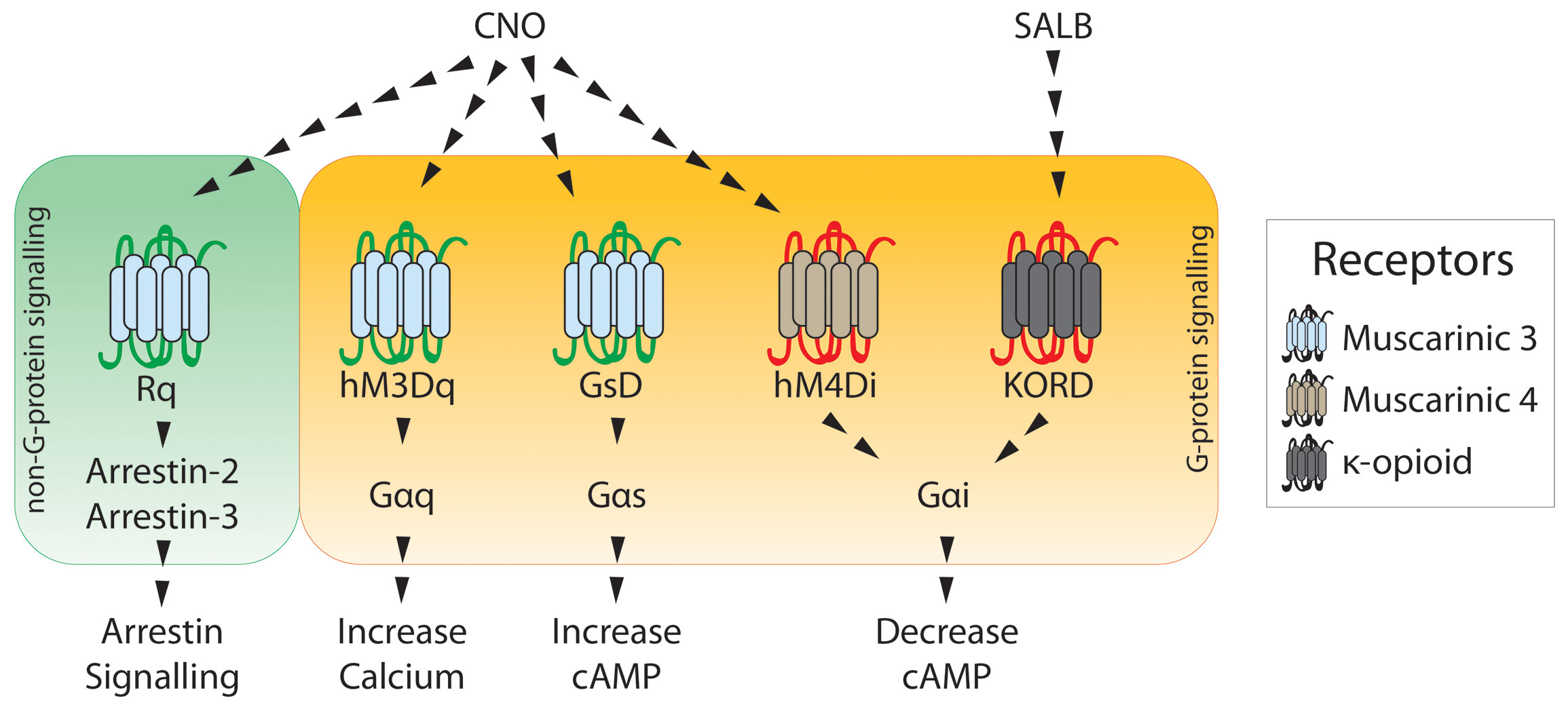
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)
DREADDs, use a modified human muscarinic receptor that no longer binds to its endogenous agonist, acetylcholine. The receptor is now only activated by binding of clozapine-N-oxide, or other actuators. This allows for selected activation or inhibition (depending on the type of DREADD being used) of genetically or projection defined subpopulations of neurons. This can be used in combination with operant conditioning to test how specific projection populations contribute to decision making and cue-reward processing.

Miniature Scope
Traditional neurotechnologies limit brain mapping to either fine detail in very few neurons at a time (microscale), or in large regions without the detail of their individual neurons (macroscale). To truly understand how the brain functions, we need technologies that can map neural circuit activity at the mesoscale, i.e in thousands of individual neurons simultaneously during a cognitive or behavioral task.
Machine Learning
Behavior is an essential component to understanding brain function. As part of our quest to better understand the brain, we use machine learning technique to help us better measure behavior data (e.g.,DeepLabCut). With advances in microscopy and machine learning, I am also interested in mapping neural/vascular networks at high resolution.
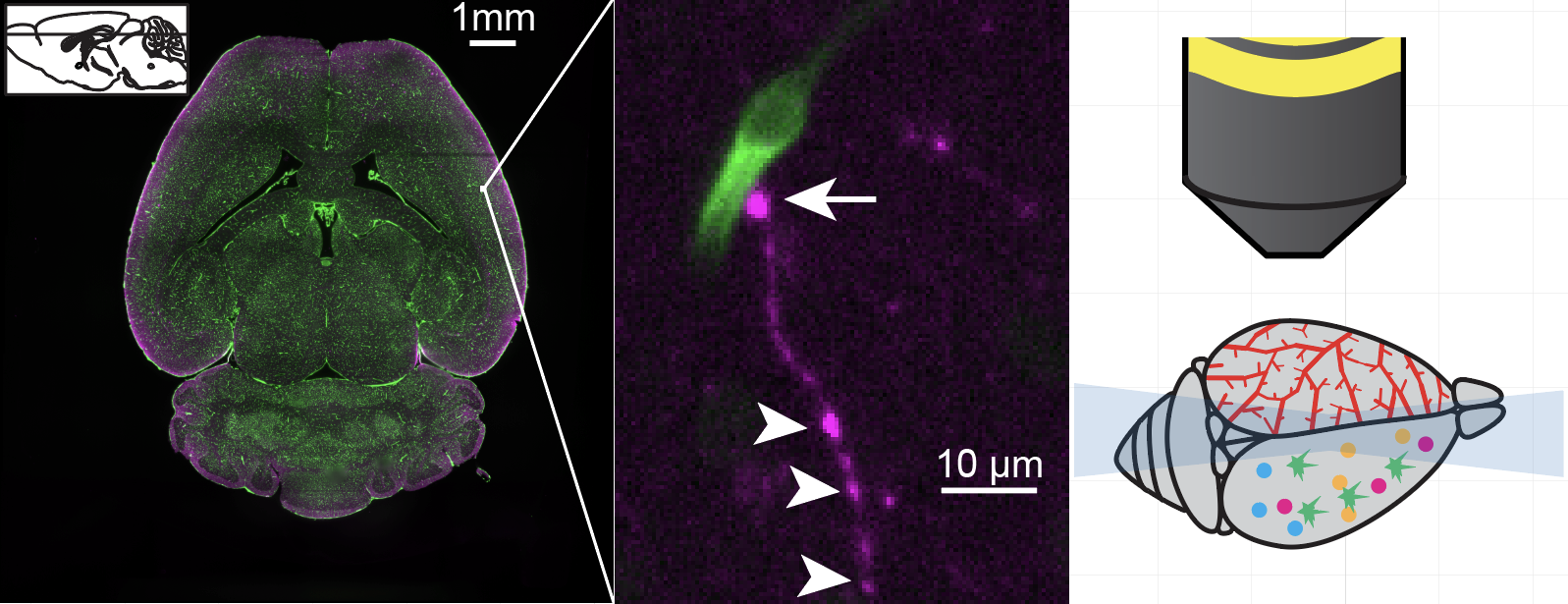
Light Sheet Flurorescence Microscopy
Cataloging brain cell types and their connectivity in normal brain is foundational to uncovering the mechanisms and therapeutic opportunities for brain disorders. Light-sheet microscopy provides a major leap forward in our capabilities for fast volumetric microscopy in diverse areas of neuroscience.
Detailed Behavior Monitoring